Pharmacy Cleanroom Airflow
Pharmacy cleanrooms are designed with highly controlled airflow systems to maintain sterile conditions and prevent contamination during the preparation of sterile compounds, such as intravenous medications and chemotherapy agents. These cleanrooms typically use laminar (unidirectional) airflow combined with HEPA filtration to create a constant stream of clean air that sweeps particles away from critical areas.
Positive pressure is maintained in most sterile cleanrooms to prevent the ingress of contaminated air from adjacent spaces, while negative pressure may be used in rooms handling hazardous drugs to protect personnel. The airflow is carefully engineered to meet ISO classification standards and USP <797>/<800> regulations, ensuring a contamination-free environment for safe pharmaceutical compounding. Proper airflow design is essential to safeguarding both patient safety and product integrity.
Airflow in a USP <797> Cleanroom
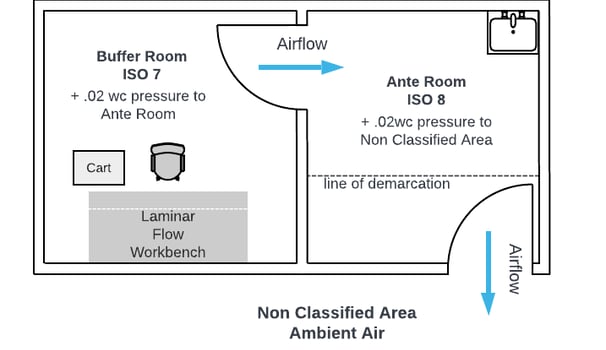
A USP <797> cleanroom is designed to support the sterile compounding of non-hazardous pharmaceuticals, with a focus on maintaining a controlled, clean environment to protect product integrity and patient safety. These cleanrooms utilize positive pressure airflow, where filtered air is continuously pushed into the cleanroom and flows outward to adjacent, less-clean areas. This prevents contaminants from entering critical compounding zones. High-efficiency particulate air (HEPA) filters supply unidirectional airflow—often in the form of laminar flow—to minimize turbulence and ensure a constant sweeping of airborne particles away from sterile work surfaces. The air quality and pressurization are carefully managed to meet ISO Class 7 or better standards in buffer areas and ISO Class 5 within primary engineering controls (PECs), such as laminar airflow workstations or compounding aseptic isolators (CAIs).
Airflow in a USP <800> Cleanroom
USP <800> cleanrooms are specifically designed for the safe handling and compounding of hazardous drugs, including chemotherapy agents. To protect healthcare workers and the environment from exposure, these cleanrooms operate under negative pressure relative to surrounding areas. This airflow design ensures that any hazardous particles or vapors are contained within the room and cannot escape into adjacent spaces. HEPA-filtered air enters the room and is exhausted either through external ducting or appropriate filtration systems. Compounding must be performed in a Class II B2 biosafety cabinet or a compounding aseptic containment isolator (CACI), located in a room with at least 12 air changes per hour (ACH). The negative pressure environment and robust filtration system ensure both containment and compliance with safety standards for hazardous drug preparation.
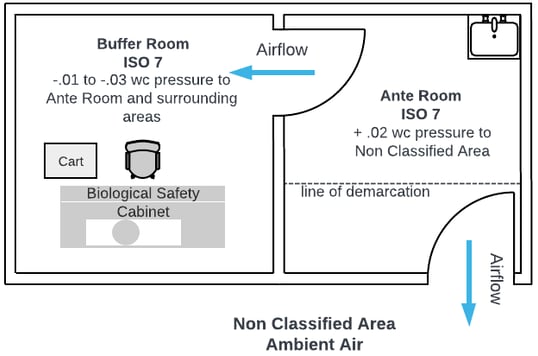
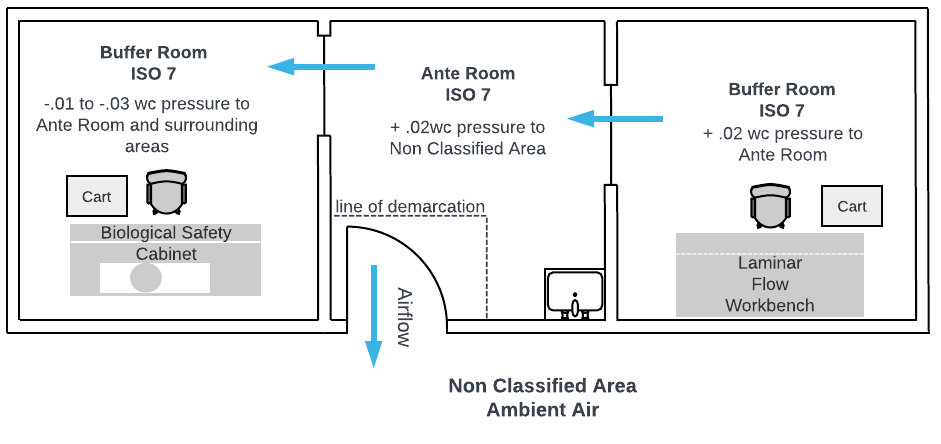
In a pharmacy cleanroom suite where a single anteroom serves both a non-hazardous buffer room and a hazardous drug (HD) room, careful airflow and pressure differentials are essential to maintaining compliance with USP <797> and USP <800>. The anteroom acts as a transitional space, supporting hand hygiene, gowning, and reducing particulate contamination between the general pharmacy and the cleanroom areas. It is typically maintained at positive pressure relative to the general pharmacy, but neutral or slightly negative relative to the hazardous drug room to prevent HD contamination from escaping. The buffer room (for non-hazardous sterile compounding) is kept at positive pressure to protect product sterility, while the HD room operates at negative pressure to contain hazardous drug vapors or particles. This configuration requires precise control of air changes per hour (ACH), pressure gradients, and directional airflow to ensure both patient and worker safety while maintaining regulatory compliance.
Contact Us for Cleanroom Certifications or HEPA Filter Changes
Need a cleanroom certification? Looking to purchase HEPA filters or request a filter change? Our team is here to help—reach out today!




